The $114B spent on biopharma acquisitions in 2025—more than doubling 2024's $46B total—revealed a singular obsession: assets with near-term revenue visibility. According to EY transaction data compiled by Arda Ural, EY Americas life sciences leader, 58% of 2025 deal value went to marketed products or Phase 3 candidates. This wasn't a bet on the future. It was a hedge against a present crisis.
The patent cliff is no longer theoretical. Industry estimates put $300B or more in branded drug revenue at risk through 2030—and the erosion has already begun. Novartis’s Entresto ($7.8B) lost exclusivity in July 2025. BMS’s Eliquis ($13B) faces approved generics in 2026.
The concentration is acute. Merck’s Keytruda alone—$29.5B in 2024 sales, representing 46% of company revenue—expires in 2028. AbbVie’s Humira franchise illustrates what awaits: revenues collapsed from $21.2B in 2022 to $9B in 2024 following U.S. biosimilar entry in January 2023.
The strategic response has been remarkably uniform: acquire assets that can contribute to the income statement within 36 months.
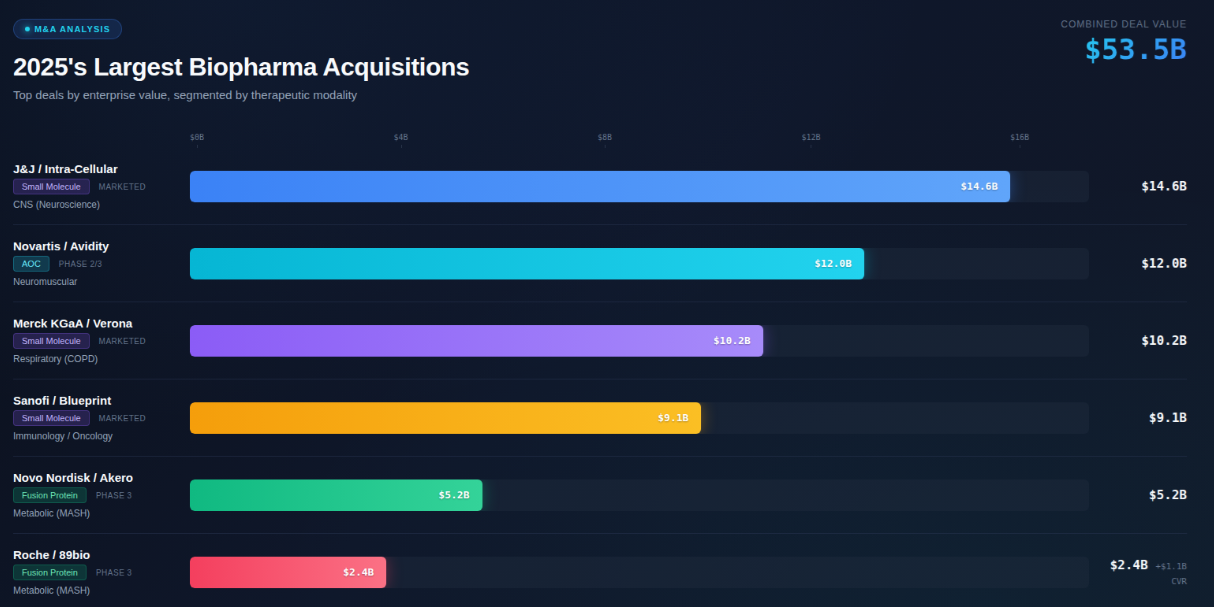
The Novartis/Avidity deal, often cited as validation of "platform convergence," was explicitly valued for its three late-stage neuromuscular programs, with platform optionality as a secondary consideration. To be clear, antibodies are the dominant modality within the biologics subset of this commercial-stage rush, reflecting their manufacturing and regulatory maturity—the error is in elevating this technical reality to the level of primary strategic intent.
CNS Overtakes Oncology: A Therapeutic Area Shift
While modality preference receded as a driver, a major shift in therapeutic area emerged. According to EY's 2025 M&A analysis, CNS/neurology captured $30.7B in 2025 M&A value, overtaking oncology's $23.5B—a notable reversal from oncology's historical dominance.
This shift reflects multiple dynamics beyond modality preference:
Unmet need creating pricing power. Psychiatric and neurodegenerative conditions affect tens of millions of patients with inadequate treatment options. Schizophrenia (2.8 million U.S. patients), major depressive disorder (21 million), and Alzheimer's disease (6.7 million) represent massive populations where new mechanisms of action can command premium pricing without the biosimilar erosion pressures facing oncology.
Blood-brain barrier as natural moat. CNS drugs require specialized delivery capabilities—whether small molecules engineered for BBB penetration (Caplyta) or antibody-oligonucleotide conjugates (AOCs) targeting transferrin receptors (Avidity's platform). This technical barrier limits competition and extends franchise value.
Regulatory tailwinds for biologics. The Inflation Reduction Act creates a structural preference: biologics receive 13 years of exclusivity before Medicare price negotiation eligibility, versus 9 years for small molecules. This asymmetry is reshaping portfolio strategy toward biologics where possible—though CNS remains volume-driven and less price-sensitive than oncology, explaining why acquirers remained willing to pay for CNS small molecules like Caplyta.
The J&J deal exemplifies this confluence: Caplyta already generated $481M in the first nine months of 2024 (+45% year-over-year), with Phase 3 expansion into major depressive disorder representing the largest commercial opportunity. J&J paid for proven revenue with optionality attached—not the reverse.
The Metabolic Surge and Radiopharmaceutical Precedent
If CNS represented the revenue urgency story, two additional clusters represented "next mega-market" optionality:
Metabolic/MASH proteins (~$8-9B): The Novo Nordisk/Akero ($5.2B) and Roche/89bio ($2.4B upfront, $3.5B with milestones) deals targeted FGF21 analogs for metabolic dysfunction-associated steatohepatitis (MASH)—a condition affecting 5-10% of U.S. adults with no approved therapies. These are recombinant proteins, not antibodies, validating the commercial opportunity in cardiometabolic disease beyond GLP-1 agonists.
Radiopharmaceuticals (2023-2024 wave signaling 2025+ dynamics): Bristol-Myers Squibb's $4.1B acquisition of RayzeBio (February 2024), AstraZeneca's $2.4B purchase of Fusion Pharmaceuticals, and Eli Lilly's $1.4B deal for Point Biopharma represent a distinct modality cluster—radioligand therapies combining antibody or peptide targeting with radioactive isotope payloads. While these deals closed primarily in 2023-2024, they signaled strategic priorities that shaped 2025's capital allocation. The logic differs from traditional biologics: isotope supply chains (particularly actinium-225) are severely constrained, creating manufacturing-based competitive moats that acquirers rushed to secure.
China's 40% and the BIOSECURE Discontinuity
Beyond modality and therapeutic area, 2025's deal flow revealed a geographic concentration that carries structural risks.
BCG's October 2025 New Drug Modalities Report documented that more than 40% of top-20 pharma deal expenditures involved assets originating in China—the highest proportion ever recorded. Jefferies data shows Chinese biotechs accounted for 32% of global out-licensing deal value in Q1 2025 alone, up from 21% in both 2023 and 2024.
This concentration reflects China's maturation from fast-follower to genuine innovator:
But 2025 may represent peak integrated China exposure. The BIOSECURE Act, signed into law on December 18, 2025 as Section 851 of the FY2026 National Defense Authorization Act, restricts federal contracting with designated "biotechnology companies of concern." While the legislation primarily targets manufacturing services (contract development and manufacturing organizations, or CDMOs, like WuXi AppTec face immediate designation risk), with phased implementation beginning 2027-2028, it signals a broader decoupling that will restructure cross-border deal flow.
The strategic calculus has already shifted. As one industry attorney observed: "When you license something, it becomes yours." Western pharma is accelerating IP licensing from Chinese biotechs while simultaneously preparing to transfer manufacturing to U.S., European, or Indian CDMOs—a bifurcation of asset acquisition and production that will increase costs and complexity through 2027.
Deal Structure Sophistication: The CVR Signal
One final pattern deserves attention: how deals were structured, not just what was acquired. Contingent value rights (CVRs) appeared in 63% of 2025 biotech M&A deals valued above $500M, with CVR components averaging 37% of total deal value according to Jefferies' mid-October 2025 analysis. The Roche/89bio structure ($2.4B upfront + $1.1B milestone-based) exemplifies this approach.
This risk-sharing mechanism complicates the "conservative retreat" narrative. CVRs enable acquirers to pursue platform bets while preserving near-term capital—sophisticated risk management rather than blanket risk aversion. Buyers aren't avoiding innovation; they're pricing uncertainty more precisely.
Media coverage of 2025 M&A coalesced around a compelling but flawed narrative: Big Pharma retreating to "validated antibody platforms" while abandoning experimental modalities. BCG's finding that antibodies accounted for over 75% of deal value from 2023-2025 appeared to confirm this interpretation.
The framing misses critical context.
First, the 75% figure applies to the biologics/new modalities category—not total M&A. When the year's largest deal (J&J/Intra-Cellular, $14.6B) is a small molecule, and three of the top six deals are small molecules or peptides, antibodies cannot dominate total deal value when small molecules like Caplyta ($14.6B) and Ohtuvayre ($10.2B) are included.
Second, "antibody" classification obscures modality diversity. Novartis paid $12B for Avidity not because antibodies are validated, but because Avidity's AOC (antibody-oligonucleotide conjugate) platform solves RNA delivery to muscle tissue—the antibody is the truck, the oligonucleotide is the cargo. ADCs similarly use antibodies as targeting mechanisms for cytotoxic or radioactive payloads. These are hybrid modalities that challenge the binary framework.
Third, the antibody concentration within biologics reflects manufacturing reality, not scientific preference. Monoclonal antibody production is mature, scalable, and globally distributed—the CDMO market is projected to grow from $21.6B to $42.7B by 2032. Gene therapy vector manufacturing remains constrained by capacity, cost, and technical complexity, with only 2-3 facilities globally capable of commercial-scale AAV production. When acquirers face 36-month pressure to generate revenue, they naturally favor modalities they can actually manufacture at scale.
The more accurate framing: Big Pharma concentrated capital in commercial-stage assets with scalable manufacturing infrastructure and established regulatory pathways—which happen to include antibodies, small molecules, peptides, and fusion proteins depending on therapeutic indication.
The "Gene Therapy is Dead" Overreaction
The Sarepta crisis of 2025—three patient deaths with acute liver failure following treatment, FDA revocation of the AAVrh74 platform designation, suspended distribution to non-ambulatory patients—generated predictable apocalyptic headlines. Combined with BCG's documentation of "stagnating growth" in gene therapy pipelines and Pfizer's decision to halt its hemophilia gene therapy Beqvez launch due to "limited interest," the narrative of modality failure took hold.
The data is more nuanced.
Safety issues are vector-specific, not modality-wide. The Elevidys deaths involved AAV-delivered gene therapy in vulnerable patient populations (non-ambulatory Duchenne muscular dystrophy patients with compromised liver function). Other vectors—lentiviral, non-viral delivery, CRISPR-based approaches—face different risk profiles. Casgevy (CRISPR-based sickle cell therapy) maintained commercial availability; China approved its first hemophilia B gene therapy in 2025.
Commercial struggles reflect pricing and infrastructure challenges, not therapeutic failure. Gene therapies priced at $2-3 million per dose face reimbursement hurdles regardless of clinical efficacy. Pfizer's Beqvez withdrawal cited "limited interest" from patients and physicians—a market acceptance issue, not a safety issue.
Venture capital hasn't abandoned the field. Kriya Therapeutics raised $320M in Series D funding in September 2025 specifically for gene therapy development, bringing cumulative funding to over $1.2B. The modality is experiencing a recalibration, not an extinction event.
The pattern resembles historical precedent: monoclonal antibodies faced an "immunogenicity crisis" in the 1990s before humanization techniques resolved the problem. ADCs experienced multiple clinical failures in the 2000s before linker chemistry improvements enabled the current generation of approved products. Gene therapy in 2025 is navigating its equivalent valley—one that may resolve through advances in vector engineering and improved immunosuppression protocols, though timelines remain uncertain.
Elevidys Q3 2025 revenue came in at $131.5M—down 27% year-over-year from Q3 2024, directly reflecting the June 2025 distribution suspension. But Sarepta maintained $500M annual guidance for the ambulatory-only population, demonstrating that even a safety-constrained gene therapy can generate meaningful revenue when addressing severe unmet need. The modality isn't failing—it's maturing through a painful but historically normal transition from platform promise to therapeutic reality.
The 2025 M&A surge reveals an industry prioritizing execution risk over scientific novelty. With 58% of deal value flowing to commercial-stage assets, acquirers paid premiums for predictable cash flows rather than platform optionality. This creates distinct positioning opportunities:
For validated protein platforms (antibodies, ADCs, bispecifics, fusion proteins): Scarcity of differentiated late-stage assets will sustain premium valuations through 2027. Companies with Phase 3 programs addressing large markets—particularly CNS, cardiometabolic, and immunology—represent the most actionable M&A targets. Expect continued 30-50% premiums for de-risked biology with scalable manufacturing.
For gene therapy and mRNA: Current valuations reflect maximum pessimism. The modality isn't failing—it's facing AAV-specific safety challenges and manufacturing scalability constraints that may be addressable, though timelines are genuinely uncertain. Patient capital willing to hold through the recalibration period may find asymmetric returns, particularly in next-generation delivery approaches (in vivo CAR-T, non-viral vectors) that bypass current limitations.
For China-originated assets: 2025 may represent peak integrated Western-China exposure. BIOSECURE implementation will force strategic restructuring—not abandonment of Chinese innovation, but shift from acquisition to licensing with manufacturing localization. Companies with established licensing infrastructure and dual-sourcing capabilities will navigate this transition more effectively. The 2026-2027 period will test whether the "license the IP, localize the manufacturing" model proves economically viable at scale.
For manufacturing infrastructure: The capacity constraints driving modality selection are structural, not cyclical. CDMOs with antibody/ADC capabilities face sustained demand; gene therapy CDMOs face near-term consolidation pressure but potential recovery by decade's end. Radiopharmaceutical manufacturing—particularly isotope production—represents the most constrained segment with pricing power.
The Macro Overlay
It's worth noting that 2025's commercial-stage preference was amplified by specific conditions: a prolonged biotech funding trough (2022-2025), elevated interest rates through H1 before September cuts catalyzed Q3-Q4 deal acceleration, and regulatory uncertainty during the FDA leadership transition. As these conditions normalize and the 2028-2030 patent cliff intensifies, acquirers may be forced toward earlier-stage assets simply because commercial-stage inventory has been absorbed.
The contrarian opportunity lies in recognizing that the market is pricing gene therapy for permanent impairment. If the modality's challenges prove to be growing pains rather than terminal—as historical precedent suggests—current valuations will appear irrational in retrospect. The question isn't whether gene therapy recovers, but when and which vectors emerge victorious.
The commercial-stage preference pattern is robust, supported by EY transaction data (58% to marketed/Phase 3) and consistent across therapeutic areas. The CNS therapeutic area emergence is documented and represents a multi-year trend. China concentration is verified (40%+ of top-20 expenditures, 32% of Q1 out-licensing) but faces structural headwinds that may reverse the pattern by 2027.
Gene therapy's trajectory remains genuinely uncertain—the modest deduction reflects legitimate debate about whether safety and manufacturing challenges are addressable on investment-relevant timelines. The 63% CVR prevalence suggests acquirers themselves are hedging these bets.
What's not in doubt: 2025's M&A surge was a defensive response to patent cliff pressure, executed through capital allocation toward assets that can generate near-term revenue regardless of modality. The "innovation renaissance" narrative overstates platform investment; the "antibody fortress" narrative misclassifies what was actually acquired. The signal is commercial-stage preference in scalable modalities. Everything else is noise.
Sources: BioPharma Dive M&A Tracker (Dec 24, 2025), BCG New Drug Modalities Report (October 2025), EY 2025 M&A analysis via Arda Ural, Morgan Stanley patent cliff analysis (July 2024), Jefferies biotech M&A analysis (October 2025), AbbVie 2024 Form 10-K, FDA press announcements, company SEC filings and press releases.
This commentary is provided for informational purposes only and does not constitute investment advice, an offer to sell, or a solicitation to buy any security. The information presented represents the opinions of The Stanley Laman Group as of the date of publication and is subject to change without notice.
The securities, strategies, and investment themes discussed may not be suitable for all investors. Investors should conduct their own research and due diligence and should seek the advice of a qualified investment advisor before making any investment decisions. The Stanley Laman Group and its affiliates may hold positions in securities mentioned in this commentary.
Past performance is not indicative of future results. All investments involve risk, including the potential loss of principal. Forward-looking statements, projections, and hypothetical scenarios are inherently uncertain and actual results may differ materially from expectations.
The information contained herein is believed to be accurate but is not guaranteed. Sources are cited where appropriate, but The Stanley Laman Group makes no representation as to the accuracy or completeness of third-party information.
This material may not be reproduced or distributed without the express written consent of The Stanley Laman Group.
© 2025 The Stanley-Laman Group, Ltd. All rights reserved.